Positive Results: The R&D Behind COVID-19 Diagnostics
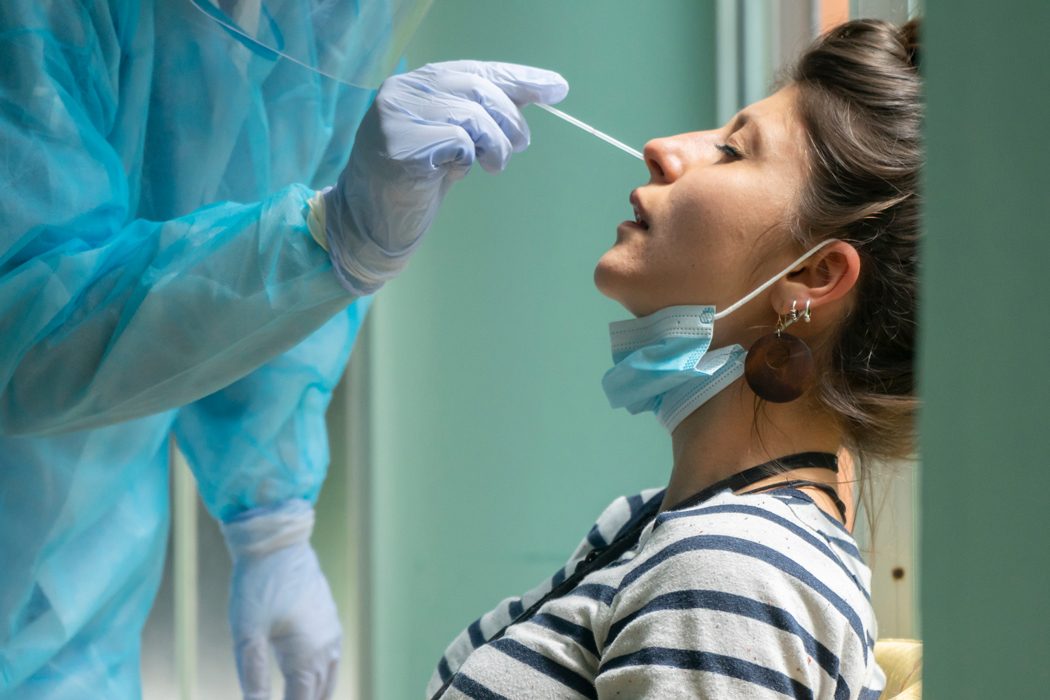
As a second wave of COVID-19 cases is being reported in Europe and the United States, we take a look at innovations in COVID-19 diagnostics and testing that could help the world beat back the spread of this infectious disease.
In Europe and the United States, a second surge of COVID-19 cases is taking place. Europe now accounts for 46% of global COVID-19 cases, and almost a third of total related deaths. In early November, the United States reported more than 126,000 new COVID-19 cases two days in a row. Nineteen states also reported a record-high number of hospitalized COVID-19 patients.
While there is no authorized COVID-19 vaccine yet, governments around the world must ramp up COVID-19 testing to curb the spread of the virus. In this article, we take a look at global initiatives to develop and ramp up more effective and expeditious COVID-19 tests.
Types of COVID-19 tests
According to the U.S. Food and Drug Administration (FDA), there are two types of COVID-19 tests:
- A diagnostic test shows if you are currently infected and should be in quarantine or isolation. There are two types of diagnostic tests: molecular tests such as the RT-PCR test detect the genetic material of the virus, while antigen tests look for specific proteins from the virus.
- An antibody test looks for antibodies, which may indicate if you were previously infected with the virus that causes COVID-19.

SOURCE: World Health Organization
Scale and speed: the race to ramp up COVID-19 testing
According to the Rockefeller Foundation, the United States needs to administer at least five million diagnostic tests daily with a turnaround time of 48 hours to effectively contain the community spread of COVID-19.
To increase the scale and speed of COVID-19 testing, the National Institutes of Health (NIH) launched the Rapid Acceleration of Diagnostics (RADx) Initiative last April. The initiative aims to “speed delivery of accurate, easy-to-use, scalable tests to all Americans.”
Proposals from all over the world undergo four phases of development:
- In process: the completed proposal undergoes initial review
- Deep dive: working with a team of experts, applicants identify risks and milestones
- Phase 1: the proposal is chosen for de-risking and validation
- Phase 2: the proposal is selected for further development, manufacturing, and distribution support
“Normally it takes five to six years to develop a diagnostic from concept to wide-scale dissemination,” says Bruce Tromberg, director of the National Institute of Biomedical Imaging and Bioengineering (NIBIB) and head of RADx’s technology division, told Discover Magazine. Through the RADx Initiative, the NIH hopes to expedite the process from years to months by processing lab-based tests with “super-high efficiency” while also introducing less sensitive, but faster tests that can be done without a lab at all.
In early October, the NIH announced a third round of COVID-19 testing technologies for scale-up and manufacturing. These six new RADx initiative contracts offer new modes of sample collection, processing, and return of results.
Innovations include integration with smart devices, a mobile processing lab that can be used in COVID-19 hotspots, and a single-use RT-PCR device. These technologies are expected to add as many as half a million tests per day to the U.S. capacity by the end of 2020.
Viral Antigen detection
Ellume USA LLC (Valencia, California)
- Type of sample: Nasal swab
- Test turnaround time: 15 minutes or less
- Two unique test cartridges contain a single-use, digital fluorescent immunoassay antigen test. One cartridge testing can be used at the point of care or in laboratory settings for higher throughput. A second cartridge is being designed for home use with a self-administered nasal swab.
Luminostics, Inc. (Milpitas, California)
- Type of sample: Shallow nasal swab
- Test turnaround time: 30 minutes or less
- A rapid, smartphone-readout, antigen immunoassay that uses glow-in-the-dark nanomaterials to sensitively and specifically detect SARS-CoV-2 from shallow nasal swabs, this test may be used first for point-of-care use and later for home use.
Quanterix (Billerica, Massachusetts)
- Type of sample: Nasopharyngeal, saliva, or self-acquired blood from a finger prick
- Test turnaround time: Collection, transport, and processing of sample will occur within 24-48 hours using existing sample collection logistics infrastructure through a network of centralized labs
- A laboratory antigen test with ultra-sensitive single-molecule immunoassay technology to enable detection from a variety of sample types.
Viral RNA detection
Flambeau Diagnostics (Madison, Wisconsin)
- Type of sample: Saliva swab
- Test turnaround time: As little as one hour
- A mobile lab van that can be deployed to screen asymptomatic individuals in schools, offices, and underserved populations, it uses new extraction technology to purify and concentrate viral RNA reliably and quickly.
Ubiquitome (Auckland, New Zealand)
- Type of sample: Nose or throat swab
- Test turnaround time: 40 minutes
- A mobile, battery-operated RT-PCR device that reports results using a proprietary iPhone app, the device can be used in urban and rural hospitals and mobile laboratories.
Visby Medical (San Jose, California)
- Type of sample: Nose or throat swab
- Test turnaround time: 30 minutes
- A palm-sized, single-use RT-PCR device that delivers highly accurate results at the point of care, the device is designed to be used by a person with minimal skills and can be adapted for testing other diseases like chlamydia, gonorrhea, and influenza.
As one of the most difficult years of the 21st century comes to a close, all our hopes are being placed on the development, approval, and distribution of COVID-19 vaccines. Until that hope becomes a reality, fast and accurate mass testing is crucial to curbing the spread of COVID-19.
As one of the Top 20 EMS companies in the world, IMI has over 40 years of experience in providing electronics manufacturing and technology solutions.
At IMI, we believe that humanity drives technology, and we direct our passion at solutions that enhance our way of living. With more than 400,000 square meters of factory space in 22 factories across 10 countries, we are positioned to build your business on a global scale.
Our proven technical expertise, worldwide reach, and vast experience in high-growth and emerging markets make us the ideal global manufacturing solutions partner.
Let's work together to build our future today.



